44 Votes Barium and sulfate ions react to form barium sulfate precipitate and the sodium and nitrate ions are unchanged - they are spectator ions. A precipitation reaction occurs upon the mixing of two solutions of ionic compounds when the ions present together in the mixture can form an insoluble compound.

How To Balance Bacl2 Na2so4 Baso4 Nacl Barium Chloride Sodium Sulfate Youtube
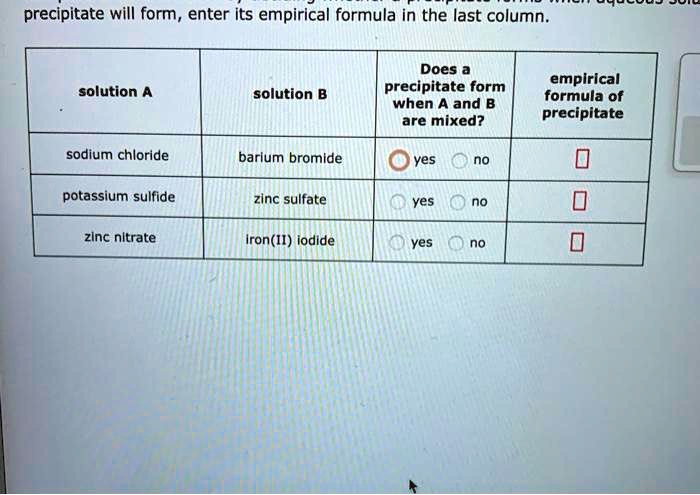
Complete the table below by deciding whether a precipitate forms when aqueous solutions A and B are mixed.

. Viruksam Marketing Your B2B Marketing Team. BaCl 2 K 2 SO 4 BaSO 4 2 KCl By examining the solubility rules we see that while most sulfates are soluble barium sulfate is not. You can however precipitate either the barium eg.
With sodium sulphate giving barium sulpate or the chloride. One could write a molecular equation showing a double. However some combinations will not produce such a product.
For reaction 2 we have Cu 2 Cl Na. Barium chloride and chromium wont give a reaction. Complete the table below by deciding whether a precipitate forms when aqueous solutions A and B are mixed.
If a precipitate will form enter its empirical formula in the last column. Sodium sulphate reacts with barium chloride to give barium sulphate and sodium chloride. In such cases the solution turns visibly cloudy a phenomenon known as precipitation.
Write the reaction and identify the precipitate. Formula in the last column. Answer 1 of 7.
Sodium chloride and barium bromide precipitate. 445 4165 Views. Nothing - barium chloride is soluble.
Empirical formula of solution A solution B precipitate sodium bromide silver nitrate yes no barium bromide potassium chloride yes Ono sodium sulfide iron II. If a solution of sodium bicarbonate is mixed with hydrochloric acid sodium chloride solution carbon dioxide and water are produced. The precipitate that was observed in the reaction must therefore be CuCO 3.
Barium chloride and potassium sulfate are both ionic compounds. When Barium bromide Solution A reacts with potassium acetate Solution B then Barium acetate and potassium bromide are formed as products. Back the boat up.
Does a precipitate form empirical solution A solution B formula of when A and Bprecipitate are mixed. What does barium chloride precipitate with. See answer 1 Best Answer.
The cloudiness is due to the formation of small aggregations of solid substance the precipitate. Complete the table below by deciding whether a precipitate forms when aqueous solutions A and B are mixed. B a C l 2 a q N a 2 S O 4 a q B a S O 4 s 2 N a C l a q Barium sulphate is insoluble in water and will precipitate as a white solid.
View the full answer. For a precipitation reaction a type of double replacement reaction to occur there must be two soluble salts which react to make an insoluble salt. 2 Na aq CO 3 2 aq Cu 2 aq 2 Cl aq CuCO 3 s 2 Na aq 2 Cl aq Note that sodium chloride does not precipitate and we write it as ions in the equation.
Does a precipitate form when A and B are mixed. Some combinations of aqueous reactants result in the formation of a solid precipitate as a product. Iron11 bromide silver nitrate barium bromide cadmium acetate.
Click to see full answer. Does barium chloride plus sodium chloride form a precipitate. The balanced chemical equation is.
Sodium sulfate solution 0. If solutions of sodium nitrate and ammonium chloride are mixed no reaction occurs. We would expect them to undergo a double displacement reaction with each other.
02 moldm 3 Potassium iodide solution 02 moldm 3 Barium chloride solution 02. They wont react with each other. Predicting Precipitates Using Solubility Rules.
A cream precipitate indicates the presence of. If you combined solutions of.

Double Displacement Sodium Sulfate And Barium Chloride Youtube

New Gcse Add L Science Ocr Gateway Sb Page 174
Solved Precipitate Will Form Enter Its Empirical Formula In The Last Column Does Solution Precipitate Form When A And B Are Mixed Empirical Formula Of Precipitate Solution B Sodium Chloride Barium Bromide Yes

0 Comments